Have you ever found yourself staring at an X-ray or CT scan, wondering how to make sense of the endless shades of gray? You’re not alone. Many students find themselves lost when it comes to interpreting radiological imaging. But here’s the thing: understanding radiology isn’t about memorizing terms or obscure concepts. There’s actually one straightforward principle that can make it all come together—something some radiologists don’t even know!
In this article, we will break down a beginner-friendly approach to interpreting X-rays and CTs. By the end, you’ll see how much simpler radiology can be.
Back to Basics: X-Ray Physics
So, what’s the big secret to mastering radiology? It all boils down to X-ray physics. Dr. Gil Chu, a respected professor at Stanford (whose brother won the Nobel Prize), highlighted a fundamental fact about X-rays: they pass through tissues at different rates based on tissue density.
The denser the tissue, the fewer X-rays pass through it, making that area appear lighter on the image. Conversely, less dense tissues allow more X-rays to pass through, resulting in a darker appearance on the scan. Simple, right? This same principle applies to CT scans, which are essentially a series of X-rays taken from multiple angles to create a 3D representation.
Think of it like this: your air-filled lungs appear dark on an X-ray because X-rays pass through them easily. In contrast, dense bones filled with calcium show up as bright white because they block more X-rays.
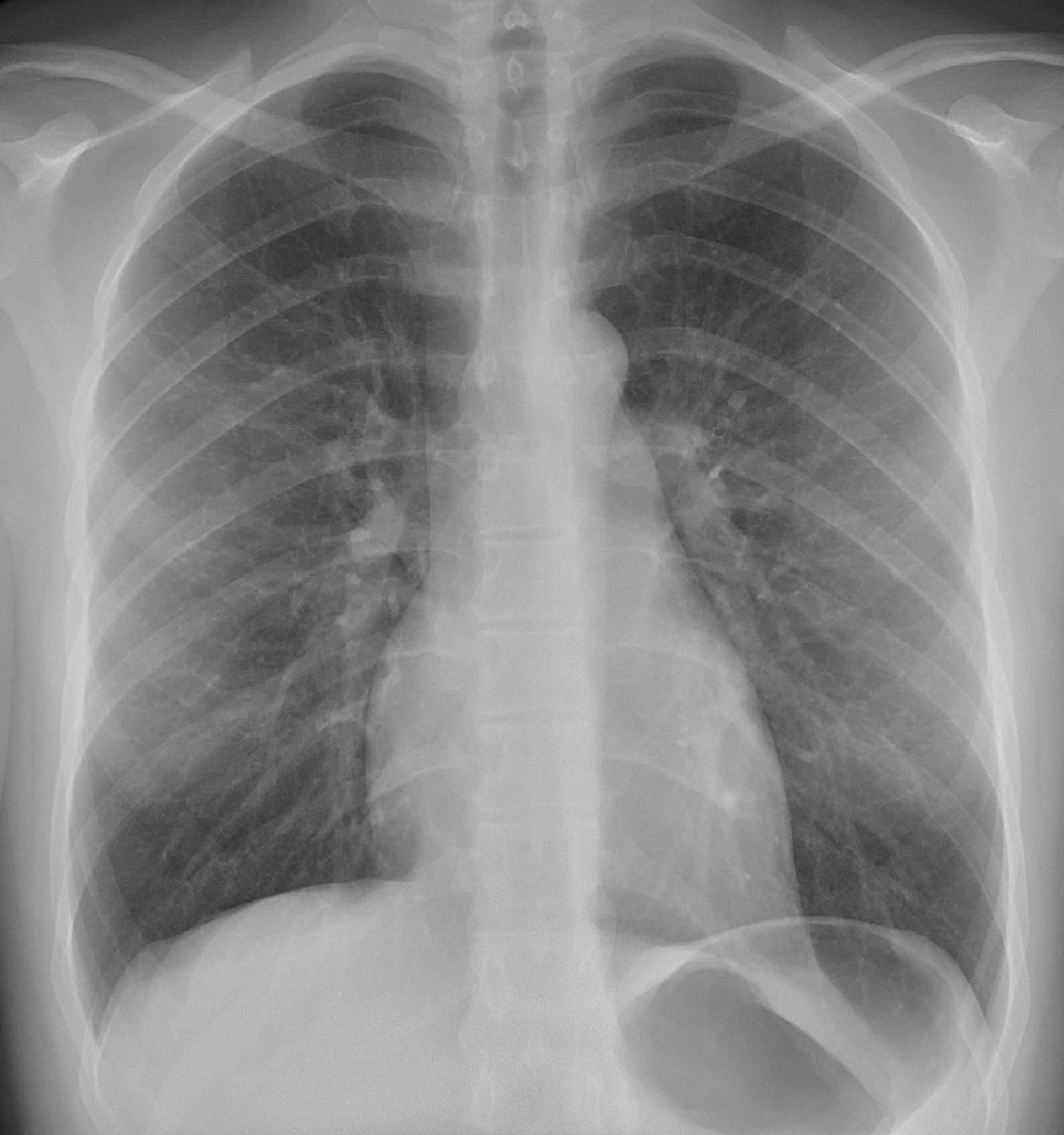
Dark regions represent air-filled lung spaces, while the ribs, dense with calcium, appear as lighter areas
The Period Table: An Unexpected Ally
We have determined that tissue density determines whether something shows up light or dark on an X-ray or CT scan, but what does this actually mean? The answer lies in nuclear density, which is influenced by how tightly packed the atoms are in a material.
The denser the atoms, the less likely X-rays will pass through them. Here’s an unexpected tip: the periodic table can help you predict how materials will appear on scans:
- Lower on the table: Higher nuclear density.
- Higher nuclear density: Brighter on the scan.
The Periodic Table in Action
Organic materials like soft tissue contain lighter elements, like carbon and hydrogen, resulting in darker appearances on scans due to their lower nuclear density. Bones, comprised primarily of calcium and phosphorus— elements lower on the periodic table— appear much brighter due to their higher nuclear density.
Blood is an interesting one. While it’s primarily organic, blood contains iron, which increases its nuclear density. This makes it appear lighter than soft tissues but not as bright as bone.
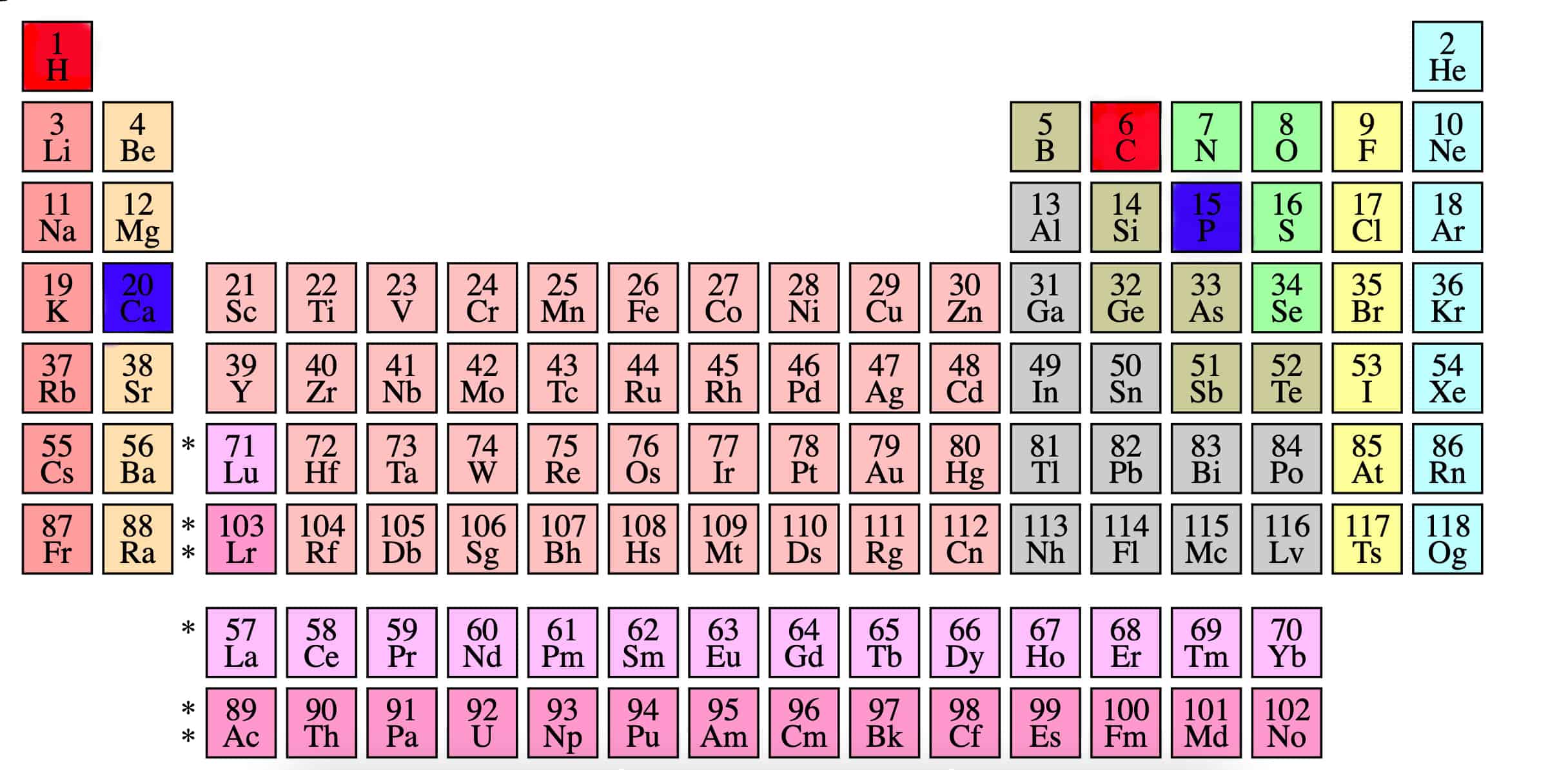
Calcium and phosphorus highlighted in blue are lower/denser than hydrogen and carbon highlighted in red
The Magic of Contrast
Now, let’s talk about contrast agents. If you’ve ever had a scan where they injected a liquid into your body, that was likely contrast material— a substance that helps certain tissues show up more clearly on the scan.
Why Use Contrast?
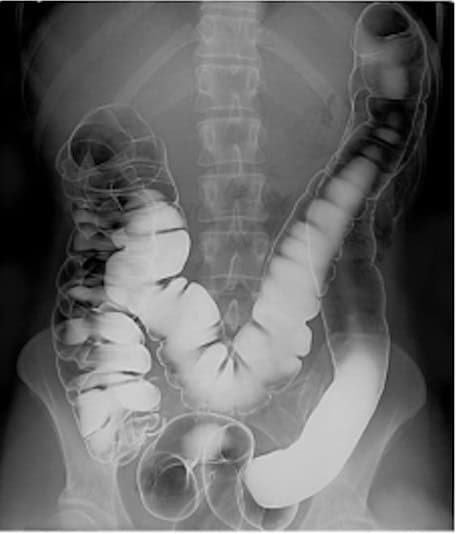
Contrast agents like barium or iodine contain elements that are low on the periodic table and have higher nuclear densities. This characteristic allows them to block more X-rays and appear bright on scans, allowing radiologists to visualize specific tissues more clearly.
But there’s a catch: you don’t always want to use contrast. Here are a few scenarios where contrast could do more harm than good:
- Fractures: Bones are already bright on X-rays and using contrast can obscure a fracture.
- Intracranial bleeding: Blood naturally appears brighter due to its iron content, so using contrast may hide critical bleeding.
- Kidney stones: Most stones are often made of calcium, so they’re already dense enough to be easily visible without contrast.
Human intestinal tract imaged via double contrast barium illustrates how contrast can enhance visibility for certain structures while obscuring others.
Conclusion
Radiology doesn’t have to be overwhelming. The secret to mastering it lies in understanding one simple idea: nuclear density determines whether something appears light or dark on an image. Once you grasp this principle, you’ll find that interpreting radiological images becomes much easier and much more intuitive.
Ready to dive deeper? Keep an eye out for more insights on mastering these fundamental principles. Remember, by focusing on core concepts rather than rote memorization, everything else will start to fall into place!